Acetonitrile vs. Methanol in Reverse Phase Chromatography
Part 1. Acetonitrile – the more expensive one
The organic solvents acetonitrile and methanol are often used as the mobile phase in reversed-phase chromatography. Commercial prices of these solvents are relatively expensive, particularly Acetonitrile for HPLC analysis. Acetonitrile seems to be used more often than Methanol in literature and conditions specified by HPLC systems manufacturers. In this article we will consider the reasons for Acetonitrile use.
Part 2. HPLC-Type Acetonitrile has less Absorbance
Figures 1 and 2 show absorption spectra for Acetonitrile and Methanol, both commercial HPLC grade solvents and special high purity grade. “HPLC grade” does not indicate that the solvent has a high absolute purity. This type of solvent is manufactured by removing impurities that have UV absorbance and the absorbance for specified wavelengths is suppressed below certain levels. It’s clear that out of the 4 solvents spectra below, HPLC-type acetonitrile has the lowest absorbance (particularly for short wavelengths).
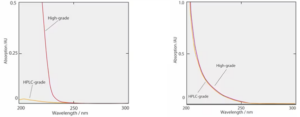
Figure 1. UV spectra for Acetonitrile solvents – Figure 2. UV spectra for Methanol solvents
Using an organic solvent with lower absorbance as the mobile phase results in less noise in UV detection, therefore HPLC-type acetonitrile is suitable for high-sensitivity analysis in the UV short-wavelength range. Also, using HPLC-type acetonitrile means that there are fewer ghost peaks for gradient baselines in UV detection. There are many other organic solvents that have a high compatibility with water but none with a lower absorbance such as HPLC-type acetonitrile.
Let’s look at an example of solvent grade related problem, which can occur in ant lab.
Analyst A measured ephedrine in ephedra at 210 nm but could only obtain data with a noise level much higher than that in the data obtained by his colleagues. He concluded that there was a problem with his analytical technique but learned, on consulting peers, that HPLC-type acetonitrile was used in previous analysis. Analyst A had thought that this was an unnecessary expense and so he used regular grade Acetonitrile. This was the problem. He had been struggling with a mobile phase giving a background level well over ten times as large. After this, they made it a rule to clearly indicate the solvent grade in every procedure.
There is no significant difference in the spectra obtained with HPLC- type methanol and special-grade methanol but the absorbance level for the special grade is not guaranteed and so there may be some inconsistency. There is not much difference in the price and so, if possible, we should use the HPLC-type.
Part 3. The Magic of System Pressure
The pressure applied to the column varies with the type of organic solvent and the mixing ratio. Fig. 3 shows some examples illustrating the relationship between the mixing ratio of solvents with water and the system/column pressure for water/acetonitrile and water/methanol mixtures. The pressure for methanol increases significantly with the proportion of water, whereas the increase for acetonitrile is not so marked. Therefore, if Acetonitrile is used, much lower pressure can be expected on the HPLC system with the same flow rate when using Methanol.

Figure 3. Organic solvent ratio and column pressure impact – C18 column 150 x 4.6mm, 5um; 1.0 mL/min
The pressure also tends to become lower as the viscosity of the solvent decreases due to the higher column temperature. Setting the column temperature between 25-40oC and comparing the column pressures for water/acetonitrile and water/methanol, causes a lowering of the overall system pressure yet still the system pressure will be higher using methanol. When switching the mobile phase from acetonitrile to methanol, the pressure resistance of the equipment and column should be verified.
Part 4. Acetonitrile has a higher elution capacity/strength (in general)
When acetonitrile and methanol are mixed with water in the same proportion (in general) the elution capacity will be higher for acetonitrile. With low mixing ratios, as seen with caffeine and phenol, the same retention times can be obtained with a proportion of acetonitrile that is much less than half that of methanol, see Fig. 4.
However, in cases where 100% organic solvents (or close to 100% solutions) are used, as seen with carotene and cholesterol, methanol sometimes exhibits a higher elution capacity, see Fig. 5.
The behavior of mixed solvents is difficult to understand but, in this case, it seems that the behavior (polarity) of the single solvent is more prominent.
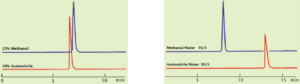
Figure 4. Caffeine elution at 1.0mL/min Figure 5. Cholesterol – same ratios (95/5)
In extreme cases, with a mixing ratio of 50 /1, for example, errors made in preparation can significantly affect retention times, and a long time may be required to reach equilibrium. If this sort of problem is experienced with acetonitrile, methanol may be a more practical alternative if analysis can be performed using it with a smaller ratio (e.g., 10 to 1).
To further understand the elution strength of acetonitrile and methanol see Fig.6 below an example of parabens separation, which is p-hydroxybenzoic acid, with C18 column (Shim-pack VP ODS).
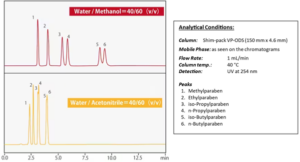
Figure 6. Parabens – elution order impacted by solvent used.
The nomogram in Fig. 7 shows the ratios of methanol and acetonitrile to water with equivalent solvent strength, useful for approximate calculation of elution strength when changing between these solvents. If we have previously been using acetonitrile as the mobile phase with a ratio to water of 50/50 (v/v), when changing to methanol the equivalent ratio of methanol/water would be 60/40 (v/v).
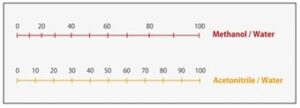
Figure 7. Acetonitrile and Methanol – elution strength nomogram
Additionally, if there are differences in the solvent temperature, measuring out solvents by weight rather than volume (taking specific gravity into account) reduces discrepancies in the mixing ratio.
Part 5. Selectivity of Separation (Elution) Is Different
The selectivity of separation differs between acetonitrile and methanol. In the example shown in Fig. 8, the elution order for phenol and benzoic acid is different in the two cases. Note that, if the proportion of water is high, phenol is eluted first with acetonitrile too. This is believed to result from differences in the chemical behavior of the organic solvent molecules. Methanol and ethanol are protic, and acetonitrile and tetrahydrofuran are aprotic solvents.
It is therefore reasonable to conclude that, if selective separation cannot be attained with acetonitrile, then analysis should be tried using methanol.
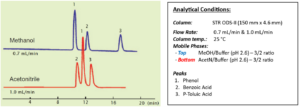
Figure 8. Acetonitrile and Methanol – selectivity of elution order
We can look at this in addition to column interactions also, as selectivity depends on the properties of the dissolved compound. It’s not the case that selectivity is always higher for one or the other solvent; there are always additional forces in play. Considering the case of positional isomers, phenyl columns may be the most appropriate amongst columns for reverse-phase chromatography. In addition to hydrophobic interactions, π-π interactions of the phenyl stationary phase contribute largely to separation. Fig. 9 shows an example of the separation of positional isomers of cresol. Acetonitrile (CH3-C≡N) has a triple C≡N bond and therefore available π electrons, whereas methanol (CH3-OH) has no π electrons. For a phenyl column, using methanol as the mobile phase allows π-π interactions of compound and column stationary phase, which improves the separation.

Figure 9. Positional isomers selectivity using Methanol and Acetonitrile – enabling π-π interaction
Part 6. Heat of reaction from mixing with water
For isocratic elution, water, and organic solvent pre-mixed in the bottle are used as the mobile phase.
When mixed with water, methanol reacts exothermically. By contrast, acetonitrile reacts endothermically and therefore the temperature of the liquid will go below room temperature. As the acetonitrile mixture gradually returns to room temperature, bubbles tend to form in the liquid. Also, if the mixture is used as mobile phase before it has returned to room temperature, retention times will be faster and only stabilize as the liquid approaches room temperature.
Meanwhile, methanol produces heat when mixed with water, which has a degassing effect. This means that preparing a water and methanol mixture as the mobile phase requires less care than in the case of acetonitrile. Consideration is therefore required for degassing.
Part 7. Summary
This completes our comparison of acetonitrile and methanol, which are most often used as the mobile phase in reversed-phase liquid chromatography. From the perspective of analytical workflow, there are differences in column pressure, UV absorption, and buffer compatibility, and when it comes to analytical separation, attention should be paid to elution power, separation selectivity and retention behavior. Understanding of these differences in chemical properties of methanol and acetonitrile, together with appropriate column combinations, reduces the risk of problems in HPLC analysis and improves the efficiency of robust method development.
We can summarize by saying that it is usually safe to use HPLC-type acetonitrile, and that methanol should be tested/verified if the selectivity or peak shape is unsatisfactory. However, it is worth bearing in mind that these two solvents explicit various properties when establishing analysis conditions.
Source: Tips for practical HPLC analysis – Separation Know-how. LC World Talk Special Issue Volume 2. Shimadzu.
Liquid Chromatography – Master the Basics
This article is part of our “Liquid Chromatography – Master the Basics” series, your go-to resource for comprehensive and insightful updates on the world of liquid chromatography. Each month in 2024 we will dive into a Liquid Chromatography topic, offering content that is both accessible to beginners and beneficial for experienced scientists.
For information on Shimadzu instrumentation:
Sebastian Jurek is an application consultant with Mason Technology with specialist knowledge in the Shimadzu range of instrumentation. He holds more than 22 years experience in chromatography techniques and analytical method development, optimisation and troubleshooting.
Get in touch with Sebastian today if you would like further information on our range of Shimadzu products.
 Sebastian Jurek
Sebastian Jurek
Application Consultant for Shimadzu Chromatography
E: sjurek@masontec.ie
M: +353 87 436 4185
T: +353 1 4154422
RECENT POST
Introducing the Eppendorf Research® plus 4-Pack with e...
Apr 24, 2024
NewsMettler Toledo Launches New Generation of Laboratory Ba...
Apr 22, 2024
NewsMason Technology is one of Ireland's Best Workplaces&#x...
Apr 18, 2024
NewsWebinar: Thinking outside the mouse: ex-vivo dissection...
Apr 11, 2024
WebinarsUnderstanding Buffers in Liquid Chromatography
Apr 10, 2024
BlogExploring Texture Analysis in advancing Medical Device ...
Mar 26, 2024
BlogFreeze Drying Adviser Vol.2: Illustrated Toolkit for Ge...
Mar 13, 2024
White PapersSolvents Mixing Ratio in Liquid Chromatography
Mar 06, 2024
Blog

