Mobile Phase Preparation – Liquid Chromatography
Mobile Phase Preparation
Aqueous solvents, organic solvents, and mixtures of these types of solvents are usually used as the mobile phase in high performance liquid chromatography (HPLC). Buffer solutions are often used as aqueous solutions. Specific preparation methods for some of the representative buffer solutions used in HPLC will be covered in a separate session. In general, however, there are many cases where the definition of buffer solutions is vague. There are also cases where, because of differences between the instructions given in documentation and the actual preparation methods used, disparities in mobile phases occur that will affect the chromatograms and the analysis results. There are many aspects of mobile phase preparation that can be thought of as blind spots. This applies not just to buffer solutions but also, for example, to solvent mixing methods. Here, using phosphate buffer as an example, we will look at the effect that the mobile phase preparation method can have on analysis results.
Part 1. Preparation of Buffer Solutions – understanding mM and pH adjustment
In general, how something described as “20 mM phosphate buffer solution (pH 2.5)” is actually prepared? We will look at several possible cases.
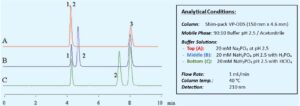
First, let us assume that we are talking about a buffer solution that uses phosphoric acid, but that the counterions are unclear. If we assume that they are sodium ions, the next problem is that we do not know whether the “20 mM” refers to the concentration of the phosphoric acid or sodium phosphate. If we think of this solution as “20 mM phosphoric acid (sodium) buffer solution”, we can consider “20 mM” to be the concentration of phosphoric acid. If, however, we consider “20 mM” to be the concentration of sodium, we can think of this solution as a “buffer solution created by the pH adjustment of an aqueous solution of 20 mM sodium dihydrogen phosphate”. The pH value of an aqueous solution of 20 mM sodium phosphate is approx. 5.0, therefore, to attain a pH value of 2.5, pH adjustment with some acid is required. Depending on the acid used for pH adjustment, the ion-pair effect may occur, and there may be some influence on the analysis results. We can see then that there are several possible interpretations for the term “buffer solution”.
Fig. 1 shows the effect on the analysis results of interpreting the above example three different ways. The top line shows the result obtained by interpreting “20 mM” as the concentration of phosphoric acid and using a solution prepared as “20 mM phosphoric acid (sodium) buffer solution (pH 2.5)” as the mobile phase. The middle and bottom lines show the results obtained by interpreting “20 mM” as the concentration of sodium dihydrogen phosphate and adjusting the pH value to 2.5 by respectively adding phosphoric acid and perchloric acid. As illustrated by dihydrocodeine in this example, there are cases where the retention time and consequently the robustness of the analysis technique are significantly affected.
Indicating the preparation method for buffer solutions so that the solution can be accurately identified helps to prevent problems resulting from differences in interpretation.
Figure 1. Influence of buffer pH adjustment method on chromatography
Part 2. Mixing Organic Solvents and Aqueous Solvents
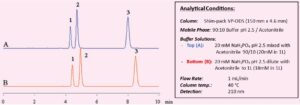
Solutions obtained by mixing organic solvents and aqueous solvents are also used as mobile phases. The way in which mixing is performed can have a significant effect on the analysis results. As an example, consider a mixture that contains 90% 20 mM Na3PO4 at pH 2.5and 10% acetonitrile. If we consider this description to indicate that the mixing ratio is 9:1, this implies that the ratio of the volume of 20 mM phosphoric acid sodium) buffer solution (pH 2.5) to that of acetonitrile is 9:1; in other words, amounts corresponding to this ratio are measured out and mixed. On the other hand, if we consider this description to simply mean “10% acetonitrile”, this implies that 20 mM buffer solution is used and is diluted with acetonitrile. Applying the latter interpretation changes the relative volumes and consequently the amount of 20 mM buffer solution is larger. There is a tendency to think that there is no significant difference between these two interpretations.
Fig. 2, however, shows how the mixing method used can have a significant effect on the chromatographic results and particularly peak retention times. In general, regarding the preparation of mobile phases for HPLC, it seems that the notation “A:B = 3:2 (V:V)”, indicating that an amount of solution A corresponding to a relative volume of 3 and an amount of solution B corresponding to a relative volume of 2 are separately measured out and mixed together, is commonly used. In practice, the total volume of the mixture will be less than a relative volume of 5.
Figure 2. Impact of Mobile Phase mixing methods on chromatography
The problems mentioned above occur not only in the preparation of mobile phases, but also in the preparation of sample solutions and other solutions. Also, different practices and conventions are used in different fields (e.g., pharmaceuticals, chemical industry), further adding to the potential causes of confusion. Official documents, such as the Pharmacopoeia documents (USP, Ph. Eur., JP), give general principles and definitions related to the preparation of solutions. It is advisable to refer to these documents and to strive on a daily basis to use notation that avoids confusion.
Source: Tips for practical HPLC analysis – Separation Know-how. LC World Talk Special Issue Volume 2. Shimadzu.
Liquid Chromatography – Master the Basics
This article is part of our “Liquid Chromatography – Master the Basics” series, your go-to resource for comprehensive and insightful updates on the world of liquid chromatography. Each month in 2024 we will dive into a Liquid Chromatography topic, offering content that is both accessible to beginners and beneficial for experienced scientists.
For information on Shimadzu instrumentation:
Sebastian Jurek is an application consultant with Mason Technology with specialist knowledge in the Shimadzu range of instrumentation. He holds more than 22 years experience in chromatography techniques and analytical method development, optimisation and troubleshooting.
Get in touch with Sebastian today if you would like further information on our range of Shimadzu products.
 Sebastian Jurek
Sebastian Jurek
Application Consultant for Shimadzu Chromatography
E: sjurek@masontec.ie
M: +353 87 436 4185
T: +353 1 4154422
RECENT POST
BUCHI release the Rotavapor R-80 System: Evaporation on...
May 03, 2024
NewsIntroducing the Eppendorf Research® plus 4-Pack with e...
Apr 24, 2024
NewsMettler Toledo launches new generation of Laboratory Ba...
Apr 22, 2024
NewsMason Technology is one of Ireland's Best Workplaces&#x...
Apr 18, 2024
NewsWebinar: Thinking outside the mouse: ex-vivo dissection...
Apr 11, 2024
WebinarsUnderstanding Buffers in Liquid Chromatography
Apr 10, 2024
BlogExploring Texture Analysis in advancing Medical Device ...
Mar 26, 2024
BlogFreeze Drying Adviser Vol.2: Illustrated Toolkit for Ge...
Mar 13, 2024
White Papers