Microscopy imaging software for FDA compliance
Is your Microscopy Imaging Software compliant with FDA 21 CFR Part 11?
PAX-it software can greatly enhance studies in labs dealing with toxicological pathology, particle sizing, quality control in the production area, thermal stage chemistry, engineering– or other aspects of pharmaceutical laboratories. High resolution micrographs can be used for documentation, reporting, or measurement and analysis in easy-to-use routines. PAX-it software enhances the workflow and the flexibility of how images are used, with capabilities for hotstage control. Image analysis, and much more.
In addition, labs required to comply with 21CFR11 security standards can take advantage of the PAX-it toolsets for Extended Security. PAX-it software is a necessary component for compliance, with a secure set of tools for tracking and locking down images, reports, and other digital assets, as well as Administration tools for setting up permissions within the software, verification of asset integrity, and audit trails.
PAX-it! Extended Security Module meets 21 CFR Part 11 requirements
For over 25 years the intuitive PAX-it framework has provided image analysis and management tools which allow you to measure and annotate images, create custom reports, and store digital assets in a secure database. Now, their additional layers of protection ensure you meet the high security standards set by the FDA.
Extended Security Features:
Administrative Tools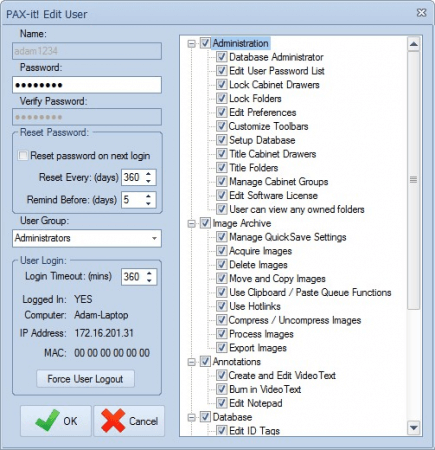
Assign unique logins to users accessing the PAX-it application. Logins may be created within PAX-it itself or sync to Windows Active Directory for a more streamlined approach to user management and authentication. An extended list of PAX-it permissions enables administrators to regulate the actions each user can take—from the assignment of user-owned folders and view-only permissions to limiting access to specific functions like annotating, calibrating and more.
Electronic Records / Electronic Signatures
Easy database storage and digital file management is the hallmark of PAX-it software. The Extended Security module brings this same ease of use to digital signatures—a core requirement for 21 CFR Part 11 compliance, but also an equally useful feature for anyone desiring greater accountability among those working with digital assets. With a button click, digital signatures are applied, locking the image, report, or data so that it may not be modified. Authentication tools are available to verify the integrity of the asset, with built-in warnings to alert customers if any original or signed file has been altered after insertion into PAX-it.
Detailed Audit Trails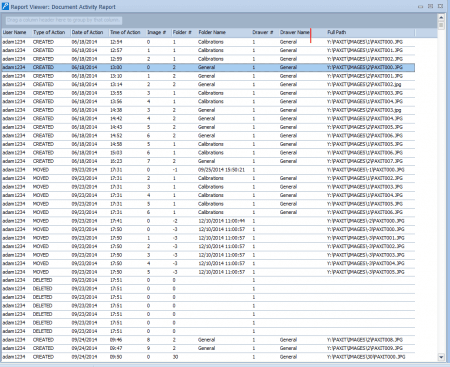
Allows administrators to track activity related to image manipulation, including moving, deleting, adjusting, etc. The creation and modification of other file types (e.g. reports, presentations) are logged. Audit trails are sortable by the image activity, by user activity, by date, and more.
For more information:
Jenny Lanigan is a senior technical sales consultant with specialist knowledge of PAX-it! products and their applications. Get in touch with Jenny today if you would like further information or to arrange to see the software in use via an online demonstration.

Jenny Lanigan
Senior Technical Sales Consultant in the Olympus & Material Science Division
E: jlanigan@masontec.ie
M: +353 87 051 3063
DD: +353 1 4154423
RECENT POST
Introducing the Eppendorf Research® plus 4-Pack with e...
Apr 24, 2024
NewsMettler Toledo Launches New Generation of Laboratory Ba...
Apr 22, 2024
NewsMason Technology is one of Ireland's Best Workplaces&#x...
Apr 18, 2024
NewsWebinar: Thinking outside the mouse: ex-vivo dissection...
Apr 11, 2024
WebinarsUnderstanding Buffers in Liquid Chromatography
Apr 10, 2024
BlogExploring Texture Analysis in advancing Medical Device ...
Mar 26, 2024
BlogFreeze Drying Adviser Vol.2: Illustrated Toolkit for Ge...
Mar 13, 2024
White PapersSolvents Mixing Ratio in Liquid Chromatography
Mar 06, 2024
Blog
